Next generation low temperature sterilizer
Designed with the Next Generation technology, the STERRAD® 100NX System is the most advanced low temperature gas plasma sterilizer from ASP:


- Large rectangular chamber and several cycle options for the most productivity
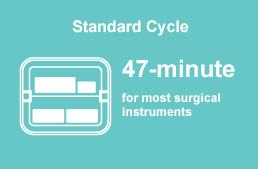
- 47-minute Standard Cycle for most surgical instruments.
- 42-minute Flex Cycle for single-channel flexible endoscopes
- 24-minute Express Cycle for fast turnaround of da Vinci® 3-D endoscopes*, rigid
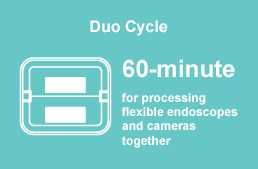
telescopes, rechargeable batteries, and many other instruments. - 60 minute DUO Cycle for processing flexible endoscopes and cameras together
- Advanced features for ease of use and quality control including large
touch-screen display, innovative foot pedal to facilitate the loading process,
hydrogen peroxide monitor to check that sufficient hydrogen peroxide is
available to assist in achieving a sterility assurance level (SAL) of 10-6,
network connectivity for remote cycle monitoring and many others. - Available as a pass-through sterilizer for the Central Sterilization Departments
with a need for separation of clean and sterile areas.
Please refer to the STERRAD® 100NX® User's guide for more detailed processing information.
* da Vinci® 3-D endoscopes are validated for use in the STERRAD® 100NX® System EXPRESS
cycle and are not validated for use in the STERRAD® 100NX® System STANDARD and FLEX
cycles.
Consult the STERRAD® Sterility Guide, www.sterradsterilityguide.com, refer
to the STERRAD® Sterilization System User's Guide or contact medical device
manufacturer for the detailed processing information.